#11 Malate / malic acid (inflammation, boosting mitochondria, reductive stress, mitochondrial NADPH / glutathione & lifespan, cancer metastasis, chemo protection)
Malate is an endogenous compound needed by mitochondria, part of the process of co2 creation & high amounts of ATP production. ATP is needed in high amounts for reactions that create the ongoing protein movements which cells need to function well. Its high in apples & supplementing malic acid has some benefits.
Unlike citric acid which can be inflammatory, malic acid has a good anti-inflammatory effect.
It causes macrophages to activate as M2 macrophages that produce less inflammatory cytokines and Il-10. (though high amounts of il-10 can become pro-inflammatory through cd8+ T cells, il-10 is needed to resolve inflammation).
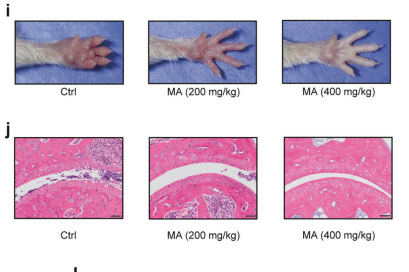
L-malate has a potent anti-inflammatory effect in an arthritis model
https://www.nature.com/articles/s41392-024-02076-9.pdf
400mg/kg mice dose outperformed 200mg/kg, and showed less than half the swelling. visually there’s a big difference.
Orally dosing rats with malic acid helped lower gouty inflammation https://biointerfaceresearch.com/wp-content/uploads/2021/06/20695837122.16821691.pdf
Small intestinal production of malate causes macrophage m2 signalling to secrete il-10 & enhance repair
https://www.nature.com/articles/s41467-023-42502-0#Sec2
In humans, less malic acid in stool was associated with worse gastrointestinal recovery.
MA content in the preoperative fecal samples was negatively correlated with the Lausanne intestinal failure estimation (LIFE) gastrointestinal injury scores post surgery
Immune cells stimulated with malic acid showed higher IL-10 expression
the recovery of intestinal injury is highly dependent on increased IL-10 production by anti-inflammatory macrophages. MA significantly increased the expression of Arg1, SOCS2, VEGFα, and TGF-β, suggesting that MA promotes the recovery of injured tissues, which is largely attributed to macrophage M2 polarization.
The upregulation of Il1b expressions in proinflammatory macrophages by an ER stressor is mainly mediated by the BiP-IRF2BP2 axis, which differs from known immunoregulatory pathways of the UPR. To note, ERS can promote BiP’s retrotranslocation from ER to cytosol
So malate stops activation of the proinflammatory macrophages induced by ER stress (Endoplasmic Reticulum stress, part of the cell that deals with protein folding and this stress is an accumulation of misfolded proteins) and increases the M2 anti-inflammatory form.
Restoring malic acid levels rescues macrophage polarization and enables intestinal mucosal recovery.
It potently upregulated the Wnt/β-catenin signalling pathway in the jejunum and increased intestinal stem cells
https://pubs.acs.org/doi/10.1021/acs.jafc.3c01332
*An acid with its low ph could be harsh directly at damaged intestines with a lacking mucus layer or after a while. But ingested malic acid gets neutralized to malate when it hits the duodenum after the stomach, as long as the amount doesn’t overwhelm the capacity in a short time.
I’m taking 600mg x2 at once in gelatin capsules with 150ml water and that seems to get neutralized to malate properly, no extra pain where I have damage in the small intestine.
Edit: Upping it to 1.8g I noticed increased burning so i think a good limit is around 1g at a time if there’s damage, maybe 20 minutes is enough for more
Some caution might be needed if someone has damage in the stomach.
*Generally to be on the safer side over time (especially if taking closer to 2 grams+) each capsule could be spaced apart
*It’s also not good to contact teeth directly, so good to make sure none is left on the capsule
*This looks best to drop during early few days of viral infections, more suited for the later part to lower inflammation response once a virus has peaked.
The mitochondrial electron transport chain is made up of a series of enzyme complexes used for the ATP creation process.
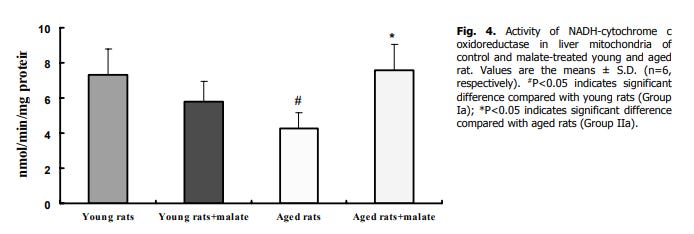
Giving aged rats 0.21g/kg L-malate orally restores their liver Complex I, III and IV enzyme activity to youth levels
https://www.biomed.cas.cz/physiolres/pdf/60/60_329.pdf
but not succinate dehydrogenase (complex II) (its favouring complex I using NADH)
increased complex I activity in both young and aged rats
Ingesting it helps use lactate better or lowers its production / increases its conversion, boosted exercise endurance and helps prevent muscle breakdown.
Malate has been suggested to increase the rate of ATP production by mitigating lactate production during states of high flux; and by doing so allowing for continued pyruvate and energy production
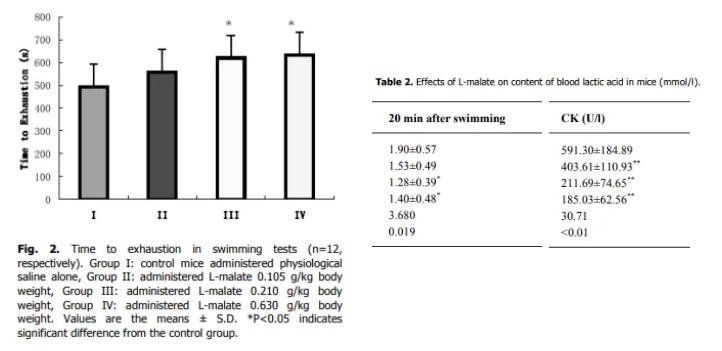
most of the effect is gained by 0.21 g/kg in mice, which might be ~1g - 1.5g for humans
increased swimming duration in mice by 26.1 % and 28.5 %, in the 0.210 g/kg and 0.630 g/kg groups. Lowered lactate & creatine kinase (marker of muscle damage) in groups 3 & 4 well
https://www.biomed.cas.cz/physiolres/pdf/56/56_213.pdf
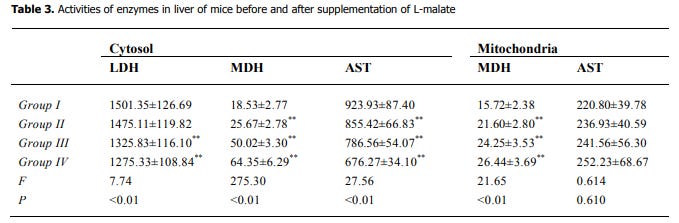
In the 1st study their Malate Dehydrogenase enzyme activity went up in both cytosol (the liquid inside the cell) and in mitochondria.
(The next part is its relevance to tumors, if this part explaining some mechanisms in poor health gets a bit wordy)
Malate dehydrogenase (MDH in the table above) either converts malate to oxaloacetate using NAD+, forming NADH, or can do the opposite and converts oxaloacetate to malate using NADH, forming NAD+.
A high NADH/nad+ ratio is a marker of poor health. Excess NADH buildup in the cytosol (reductive stress) is a sign the mitochondria complexes are lacking ability to process electrons well, causing an electron buildup & leakage. The leaked electrons react with oxygen, generating ROS like hydrogen peroxide and others which cause oxidative stress local to mitochondria and further into the cell. High NADH in the cell causes inhibition of enzymes needed for the TCA cycle to function.
If catalase & glutathione are overwhelmed (not able to deal with the high amounts of ROS) the ROS can run wild stripping electrons from DNA, polyunsaturated fats, enzymes and other proteins, damaging them.
This can also start an inflammatory cascade with the ROS acting as a signal for immune cells like M1 macrophages and neutrophils to infiltrate, release degrading enzymes, more ROS, pro-inflammatory cytokines (signalling proteins for immune cells), and signal apoptosis (programmed cell death), which can become chronic in a cycle of inflammation.
Raising malate seems counter intuitive in poor health (giving mitochondria / cells more ability to raise NADH when electrons aren’t processing properly).
But in the aged animals with lowered mitochondria complex activity, giving them malate restored it, including complex I (which processes NADH).
And below it lowered Reactive Oxygen Species / oxidative stress (ROS stress) in their heart & liver. Suggesting a restored ability for the electrons to process better, reducing electron leak and ROS formation.
https://www.biomed.cas.cz/physiolres/pdf/57/57_261.pdf
It also gives a protective effect by increasing potential to restore reduced glutathione with an nadph increase, allowing the complexes to rebuild by preventing ongoing damage.
(malic enzyme converts malate to co2 and NADPH, when malate isn’t converting to oxaloacetate to form more NADH. and NADH itself can be converted to NADPH).
Supplying mitochondria with more malate increases oxidative phosphorylation
https://pubmed.ncbi.nlm.nih.gov/2368180/
[malate regulation of oxidative phosphorylation in brain mitochondria]
https://www.sciencedirect.com/science/article/abs/pii/0003986186902407
https://pubmed.ncbi.nlm.nih.gov/24716714/
[Malate raises reduced GSH (reverses oxidized GSH) local to mitochondria, preventing the S-nitrosylation {or S-glutathionylation} of proteins needed for ATP production. allowing for more ADP supply used to create ATP at the last step after complex IV, and exporting ATP for use by cells.
Activation of mitochondrial respiration by respiratory substrates leads to increased NAD(P)H and GSH levels, which in turn reduces mitochondrial S-nitrosylated proteins.
Several mitochondrial proteins were identified as targets of S-nitrosylation including adenine nucleotide translocase (ANT) and voltage-dependent anion channel (VDAC), important components of the mitochondria permeability transition pore (MPTP), as well as ATP synthase.
ANT is responsible for the exchange of ADP and ATP across the inner mitochondrial membrane, directly controlling the supply of ADP for ATP synthesis and the export of newly synthesized ATP to the cytosol.
Glutathionylation of mitochondrial proteins is a major consequence of oxidative stress, and respiratory substrates are key regulators of mitochondrial redox status (as reflected by thiol/disulfide exchange) by maintaining mitochondrial NADPH levels.
The S-nitrosylation of ATP synthase by GSNO was found to inhibit its activity.
S-glutathionylation (by oxidized glutathione GSSG) also inhibits the ATP synthase activity, similar to nitrosylation. Our findings suggest that ATP generation during oxidative stress will be limited due to these oxidative modifications on complex V; conversely, reversible S-nitrosylation and S-glutathionylation of ATPase may temporarily protect the complex against irreversible oxidative modifications.Supplementation of isolated brain mitochondria with malate significantly increases mitochondrial NADH and NADPH levels. This rise in NADPH is crucial because it is used by glutathione reductase to convert oxidized glutathione (GSSG) back to its reduced form (GSH). Mitochondria energized with malate and ADP show a dramatic increase in GSH levels, while GSSG and S-glutathionylated proteins become nearly undetectable.
https://pmc.ncbi.nlm.nih.gov/articles/PMC3000945/
Treatment of the brain mitochondria with H2O2 resulted in a rapid drop in GSH levels and increase GSSG levels. H2O2 had no effect on GSH and GSSG levels of respiratory substrate-supplemented mitochondria.
ATP synthase appears to be the major mitochondrial protein that becomes glutathionylated under oxidative stress conditions.
The redox potential of energized brain mitochondria was calculated as −291 mV, a calculation based on GSH and GSSG values at 30 min of incubation with malate/glutamate (Fig. 2). *It may be surmised that energized mitochondria are in a more reduced state (from −171 to −291 mV).
(NADPH can be used to create more ROS through NADPH oxidase instead, which can be dysfunctionally active e.g in diabetes, and the NNT enzyme that converts NADH to NADPH can reverse in situations of high energy demand where NADH needs aren’t being met https://www.cell.com/cell-metabolism/fulltext/S1550-4131(15)00337-X
If there’s an abundance of oxidized glutathione it can be used to replenish reduced glutathione. The lowering of elevated ROS in the heart earlier indicates giving them malate preferentially replenished reduced glutathione in tissues).
So while high NADH in the cell can indicate dysfunction, well activated mitochondria are supposed to be in a reduced state with a high amount of GSH local to mitochondria (high GSH:GSSG ratio).
(an abundance of mitochondrial NADPH = ability to continually regenerate glutathione local to the chain for protection, otherwise the chain is inhibited by S-nitrosylation & S-glutathionylation).
(in resting skeletal muscle, the cytosolic NAD+/NADH ratio is estimated to be around 540, while in mitochondria it is approximately 6.3 https://pmc.ncbi.nlm.nih.gov/articles/PMC8869961/)
* This also connects to why carbs increase conversion of T4 → T3, enough NADPH in mitochondria acts as an OK signal for thyroid hormone to stimulate respiration, glucose raises NADPH allowing for regeneration of reduced glutathione when needed, giving the go-ahead for T3 to rise.
(*its more direct to NADPH than glutathione, the capacity to restore it rather than the actual raising of it)
Part of NADPH comes from glucose, part from NADH through NNT,
”In mouse brain, Nnt catalyzed NADPH generation contributes to about 50% of the total mitochondrial NADPH pool” https://pmc.ncbi.nlm.nih.gov/articles/PMC4116450/
The glutathione pool in mitochondria is supposed to be very reduced
https://moodle2.units.it/pluginfile.php/249665/mod_resource/content/1/Glutathione.pdf
Both the matrix and the IMS glutathione pools have been investigated with roGFP-based sensors. Interestingly, the matrix and IMS glutathione pools were found to be extremely reduced with EGSH values -300 mV in both compartments in yeast and mammalian cells (33, 44, 46, 68, 90)
In aging there’s generally a shift towards a more oxidized state in mitochondria
NADPH generally declines a lot during aging
https://pubmed.ncbi.nlm.nih.gov/24655393/
mitochondrial GSH:GSSG ratio generally lowers in aging and restoring this increases lifespan
https://pmc.ncbi.nlm.nih.gov/articles/PMC2585506/
The GSH:GSSG ratio, which is the primary determinant of the cellular redox state, becomes progressively more pro-oxidizing during the aging process due to an elevation in the GSSG content and a decline in the ability for de novo GSH biosynthesis
Over-expression of GCL has been shown to prolong the life span of Drosophila by up to 50 %, suggesting that perturbations in glutathione metabolism play a causal role in the aging process.
GSH:GSSG couple is considered to be the primary intracellular determinant of the redox state, because it is 3 to 4 orders of magnitude more abundant than the other redox couples and because it has low standard redox potential.
The reduction/oxidation reactions of glutathione involve two electron transfers: This also underlies the main difference between glutathione redox couple and the others, all of which involve one electron transfer reactions.
In mice, the GSH:GSSG ratios were much higher in mitochondria: liver,162; kidney, 323; heart, 25; brain, 564; eye, 314, and testis, 319. During 4 to 26 months of age (Fig. 4), Mitochondrial GSH:GSSG ratios decreased significantly with age in all tissues, except the brain.
Mitochondrial glutathione redox potentials declined (Fig. 5), that is, they became more oxidizing, or less negative, with age, ranging from 4.5 mV in the brain to 15 mV in the heart. Decreases in other tissues ranged between 9 and 12 mV.
Such data unequivocally demonstrated that aging is associated with a significant pro-oxidizing redox shift in all the organs of the mouse examined. The proximate cause seemed to the increase in GSSG content and a decrease in GSH content in some tissues.
mitochondrial GSH dropped 9% - 19% during aging, but oxidized GSSG went way up, causing a big shift lower in GSH:GSSG ratio
To determine whether the glutathione redox state is correlated with life expectancy of mice, we compared GSH and GSSG contents in mitochondria and tissue homogenates between two different strains of mice, C57BL/6 and SAM (P8) (senescence-accelerated mouse); the latter has a 30% to 50% shorter life span than C57BL/6 mouse [27]. Although the mechanism underlying the short life span of the SAM mouse is not well understood, this strain exhibited a relatively higher level of oxidative stress, indicated by the GSH:GSSG ratios, than the C57BL/6 mouse.
overexpressing a NADPH generating enzyme in flies also increases lifespan https://pubmed.ncbi.nlm.nih.gov/18809674/
In virtually all tissues, GSSG concentration is elevated during the last trimester of life. This is accompanied by an increase in the rates of mitochondrial superoxide and hydrogen peroxide production
*Note: antioxidants are not enough to increase mitochondrial GSH:GSSG ratio, where it obviously matters most for protecting mitochondria. a diet with polyphenols did
Diet I was enriched with vitamin E, vitamin C, L-carnitine, and lipoic acid, while Diet II included vitamins E and C, and 13 additional ingredients containing micronutrients with bioflavnoids, polyphenols,and carotenoids.
Both diets had a reductive effect in plasma, but only Diet II induced an increase in mitochondrial GSH and a decrease in GSSG content, and consequently a proreducing shift in GSH/GSSG redox couple. The effects on redox state in the tissue homogenates were insignificant.
I’ve seen this before where Vitamin E failed to protect well against aluminium toxicity, but Rosmarinic acid (in lemon balm) was protective.
In fibromyalgia magnesium malate lowered scores from 19-6 to 6-5 by 8 weeks https://www.tandfonline.com/doi/abs/10.3109/13590849208997961
After treating 15 FM patients for an average of 8 weeks with an oral dosage form with dosages of 1200–2400 mg of malate and 300–600 mg of magnesium, the tender point index (TPI) scores (x±) were 19–6±2–1 prior to treatment and 8±1.1 and 6–5±0.74. respectively, after an average of 4 and 8 weeks on the magnesium malate combination (p < 0.001). Subjective improvement of myalgia occurred within 48 h of supplementation. in six FM patients, following 8 weeks of treatment, the mean TPI was 6.8 ±0.75. After 2 weeks on placebo tablets, the TPI values increased to amean ± SE of 21.5 ±1–4(p < 0.001). Again, subjective worsening of muscle pain occurs within 48 h of placebo administration
600mg repeated x2 a day for 4 weeks wasn’t enough, increasing up to 1.2g twice a day was
https://pubmed.ncbi.nlm.nih.gov/8587088/
No clear treatment effect attributable to Super Malic was seen in the blinded, fixed low dose trial. With dose escalation and a longer duration of treatment in the open label trial, significant reductions in the severity of all 3 primary pain/tenderness measures were obtained without limiting risks.
Though it doesn’t show a helpful effect on tumor growth, it shows a great effect on lowering metastasis (spread) of a highly aggressive metastatic form of breast cancer.
L-malate didn’t change tumor growth, but prevented breast cancer metastasising to the lungs well. This was given as an i.p injection, oral bioavailability should be high.
Exogenous malate and α-KG exerted similar effect on HIF-1α in breast cancer cells to ME2 knockout or knockdown.
Treatment with malate (50mg/kg, i.p) significantly decreased 4T1 breast cancer lung metastasis. https://www.researchgate.net/publication/348261020_Mitochondrial_Malic_Enzyme_2_Promotes_Breast_Cancer_Metastasis_via_Stabilizing_HIF-1a_Under_Hypoxia
* Note this might be specific to certain types. It gets complicated between different environments.
e.g Succinate can promote lung cancer metastasis where there’s high SUCNR1 expression, through the SUCNR1 activating hif-1a and il-6 in tumor associated macrophages (TAMs can have mixed hybrid activation states I guess skewed a lot more than the typical M1 or M2 profile).
Though in a liver cancer experiment where there’s low SUCNR1 succinate actually created tumor regression.
In breast cancer il-6 didn’t effect migration, IL-10 lowered migration https://link.springer.com/article/10.1007/s00262-017-2106-8
& higher il-10 expression in peoples breast tumors was associated with less invasion
https://pubmed.ncbi.nlm.nih.gov/24796749/
il-10 was associated with increased survival & long term survival after resolution
https://pubmed.ncbi.nlm.nih.gov/25129439/
though here il-10 correlated with lower survival (odd as the areas with higher il-10 had lower proliferative marker)
https://bmcresnotes.biomedcentral.com/articles/10.1186/1756-0500-5-110
And though malate didn’t effect tumor size above at the amount given, 4TI breast cancer at least has shown to be responsive to il-10 https://pmc.ncbi.nlm.nih.gov/articles/PMC8072451/#s3
https://pubmed.ncbi.nlm.nih.gov/39244897/
More metastasis in colon carcinoma as il-10 decreased
https://pubmed.ncbi.nlm.nih.gov/20037839/
In a trial people with renal cell carcinoma, melanoma and lymphoma responded to il-10, pancreatic and ovarian also had a high response rate
https://www.annalsofoncology.org/article/S0923-7534(19)64273-0/fulltext
https://pmc.ncbi.nlm.nih.gov/articles/PMC10524969/
*BUT opposite effects in lung cancer (adenocarcinoma) was shown
https://pubmed.ncbi.nlm.nih.gov/23743567/
*Unless the lung tumor is metastasised from melanomas, here it worked well again
“Interleukin-10 Inhibits Tumor Metastasis Through an NK Cell-dependent Mechanism”
https://pmc.ncbi.nlm.nih.gov/articles/PMC2192723/
https://pmc.ncbi.nlm.nih.gov/articles/PMC4322764
IL-10 is now recognized not only as the most potent anti-inflammatory cytokine but also for enabling cancer immune surveillance and tumor rejection
In contrast to IL-10 competent mice that have prolonged intra-polyp cytotoxicity and never developed invasive lesions, IL-10 deficient mice rapidly lost their intra-polyp cytotoxicity and their lesions progressed into large invasive cancers
Counter-intuitively considering its anti-inflammatory effect, the positive effect relies on having good presence of T cells or NK cells https://onlinelibrary.wiley.com/doi/10.1002/bies.201300004
Here in spontaneous mice breast tumors T cells weren’t needed for the metastasis preventing effect, the IL-10 impact was from NK cells
https://academic.oup.com/jnci/article-abstract/88/8/536/915125
In spite of the more limited efficacy of IL-10 against tumor growth in immunocompromised mice, spontaneous metastasis of 410.4-IL10 cells in C.B-17/IcrCrl-SCID/BR mice was inhibited by 90%. When NK activity was suppressed by asialoGM1 ganglioside antibody in BALB/cByJ mice or in C.B-17/IcrCrl-SCID/Beige mice, the antimetastatic effect of IL-10 was lost.
This effect thus appears to be relatively independent of T-cell function but is dependent on NK activity. In contrast, the inhibitory effect of IL-10 on tumorigenicity relies on T-cell function.
Maybe malate is being depleted here, and restoring it allows for proper il-10 expression to prevent metastasis in specific tumor environments.
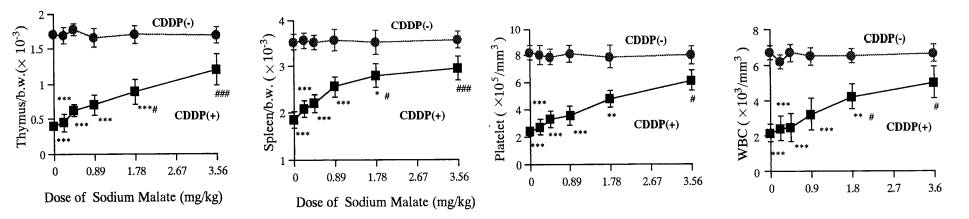
Surprisingly, a very low oral dose of 3.6mg/kg sodium malate reduced a large amount of platinum toxicity used for chemo (CDDP).
https://pubmed.ncbi.nlm.nih.gov/7697773/
Intuitively it doesn’t seem right how such a small amount orally could give such great protective effects. I figured it might be interfering with the drug absorption given simultaneously. But they were given apart by different routes with the malate 30 minutes before, and it only mildly impaired the effect (or basically not at all with the other doses)
It was enough to prevent them all dying until the higher doses of CDDP / platinum.
The researchers went further and showed orally or i.p didn’t make a difference for the effect.
Curious how such a small amount could create this protection, most or all of that sodium malate turns into malic acid in the stomach by reacting with stomach acid. Some fruit / juice can have hundreds of milligrams or grams of malic acid.
This dose is specific to this use case not creating general effects,
the platinum used for the tumor effect interacts with malate in blood (about 40% of it), forming DPM (squares below) which has similar effects with less toxicity than the usual form (circles)
https://www.jstage.jst.go.jp/article/bpb1993/21/2/21_2_121/_pdf/-char/en
administering the sodium malate at the same time or 30 minutes before had the best protective effect.
10.68mg/kg was tested with a different form of platinum (CBDCA, harder to interact with) and didn’t show the protective effect
https://www.jstage.jst.go.jp/article/bpb1993/21/1/21_1_34/_pdf/-char/en
The researchers tested a bunch of different herbs for their protective effect. at typically used amounts (1), sodium malate outperformed.
Malate has strong effects on aluminium too.
When plants take on an aluminium burden they secrete malate https://pubmed.ncbi.nlm.nih.gov/31090277/
In plant species such as wheat that do not accumulate Al in foliar tissues, it is proposed that Al is detoxified by the release of organic anions, particularly malate
Delhaize et al. (1993b) showed that Al-induced release of malate is the major mechanism of Al resistance in wheat, with the secretion of malate from wheat roots occurring within 15 min of exposure to Al3+. Furthermore, malate excretion increases with increasing Al concentration https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2017.01377/full
Regardless of whether the Al-malate complexes within the apoplast form within the root or within the rhizosphere, it is known that Al-malate complexes are non-toxic (or at the least, substantially less toxic) than is the free Al3+ ion
I’ll write about its effects in a post about aluminium & protecting against it coming soon.
It has a blood thinning effect, effective at preventing blood clots
Malic acid + citric acid + succinate combo outperformed aspirin for survival given before inducing a blood clot (might increase risk of bleeding combined with other blood thinners)
https://pubmed.ncbi.nlm.nih.gov/23913658/
malic acid works alone in vitro https://pubmed.ncbi.nlm.nih.gov/23447108/
Its more relevant for ADP induced clotting inhibition than thrombin (ADP induced clotting signals thrombin for more potent clotting, so more relevant for prevention than eliminating an existing clot)
600mg/kg in mice lowered gaba in cerebrum and increased learning ability https://pubmed.ncbi.nlm.nih.gov/9863221/
I don’t notice any gaba lowering or pro glutamate effect functionally from 1.2 grams. But there’s potential for lowering gaba excessively if dosing too high.
(& high amounts could potentially overwhelm the bodies ability to neutralise the acid to malate when it hits the small intestine)
The d-form is not the same as the l-form of malate, using high dose d-malate showed more detrimental effects https://pubmed.ncbi.nlm.nih.gov/38253688/
maleic acid isn’t malic acid / malate
Malate can help resolve inflammation and stimulate repair, help restore mitochondrial activity, protect mitochondria & cells by replenishing GSH to sort ROS, inhibit metastasis well in some cancer environments, and protect against toxicity of some metals.





















Great deep dive here cs! I eat one big Cosmic Crisp apple a day. Do you think that approaches the 600mg supplemental level you’re at? Or maybe you get lots of food source malic acid but you also supplement the 600mgx2 on top of the OJ cherries apples you might be eating? 🍓🍇😎
Also… do you think magnesium malate is beneficial? I’ll look into it…
Also the image of the arthritic mouse’s hand! Such a dramatic recovery!
Cheers! 🖐️